In brief
Inaccurate and unbalanced comparative claims that don't reflect the overall picture can cause big headaches, even where those claims have some support. Senior Associate Adrian Chang explains.
Pain is big business in Australia, and the makers of Panadol and Nurofen are constantly trying to get the upper hand. Hence Reckitt Benckiser's late 2015 comparative advertising campaign claiming that Nurofen was faster and more effective at relieving headaches than GlaxoSmithKline's Panadol.
The campaign has been held to have been misleading (GlaxoSmithKline Australia Pty Ltd v Reckitt Benckiser (Australia) Pty Ltd (No 2) [2018] FCA 1), in not accurately representing what's known about the relative efficacies of ibuprofen (the active ingredient in Nurofen) and paracetamol (the active ingredient in Panadol).
The below image captures the core message in Reckitt's advertising campaign.
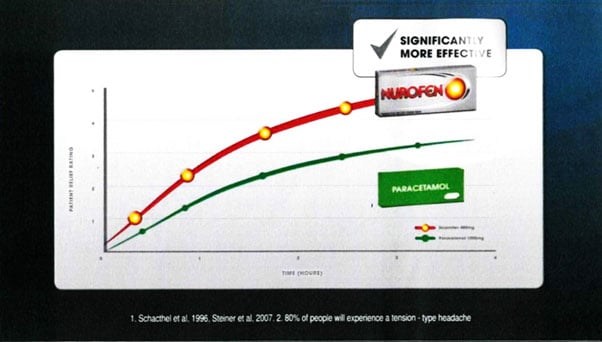
Reckitt's claim that Nurofen was superior to Panadol (and paracetamol generally), relied only on the study quoted in the advertisement, referred to as the 'Schachtel Study'.
GSK made a raft of complaints, but it all boiled down to one issue – did the advertisement, and the Schachtel Study, accurately reflect the state of scientific knowledge about the relative efficacy of ibuprofen vs paracetamol?
The graph set out in the advertisement was essentially a replica of a graph from the Schachtel Study, and at that level, the advertisement accurately represented that study's results. Further, the study concluded that a 400mg dose of ibuprofen was significantly more effective than a 1000mg dose of paracetamol (the standard recommended doses for Nurofen and Panadol) in treating muscle contraction headaches.
GSK didn't dispute the design, or the conclusions, of the Schachtel Study. Instead, it argued that the advertisement was misleading because, by focusing on only that study, Reckitt ignored other scientific studies that found no clinically significant difference between a 400mg dose of ibuprofen and a 1000mg dose of paracetamol in treating pain.
Reckitt acknowledged that those other studies didn't replicate the Schachtel Study results, but claimed they didn't contradict it either.
Reckitt's 'no evidence to the contrary' argument ultimately didn't fly with Justice Foster, who held that, despite there being no studies that were necessarily inconsistent with the Schachtel Study, the advertisement didn't convey the true state of the science.
His Honour held that the preponderance of studies had found there was, in fact, negligible difference in pain relief between ibuprofen and paracetamol. Accordingly, it was misleading for Reckitt to claim that Nurofen provided faster and more effective relief from common headaches than did Panadol, when the Schachtel Study was the only one supporting such a clear-cut claim.
This case's outcome is a reminder that comparative advertising remains tricky in Australia. It's not enough to say your specific express messages are supported – to avoid a misleading representation, statements should be balanced, accurate and reflect the overall picture. As Reckitt learned, failure to observe that standard can lead to pain from which there is no relief.



